Which Statement Best Relates Why These Animals Have So Much Blubber?
- Inquiry article
- Open Access
- Published:
From low to high latitudes: changes in fatty acid desaturation in mammalian fatty tissue suggest a thermoregulatory role
BMC Evolutionary Biology book nineteen, Commodity number:155 (2019) Cite this commodity
Abstract
Background
Nigh fatty acids (FAs) making upwards the adipose tissue in mammals have a dietary origin and endure piffling modification when they are stored. However, we propose that some of those FAs, specifically those that tin be synthesised or modified past mammals, are also beingness influenced by thermal forces and used as part of the mechanism to regulate core body temperature. As FA desaturation increases, adipose tissues can reach colder temperatures without solidifying. The ability to absurd the superficial fat tissues helps create a thermal gradient, which contributes to body rut loss reduction. Therefore, information technology is expected that animals exposed to colder environments will possess adipose tissues with higher proportions of desaturated FAs. Hither, through a model selection approach that accounts for phylogeny, we investigate how the variation in FA desaturation in 54 mammalian species relates to the thermal proxies: latitude, physical environment (terrestrial, semi-aquatic and fully-aquatic) and hair density.
Results
The interaction between the environment (terrestrial, semi- or fully-aquatic) and the breadth in which the animals lived explained all-time the variation of FA desaturation in mammals. Aquatic mammals had higher FA desaturation compared to terrestrial mammals. Semi-aquatic mammals had significantly higher levels of desaturated FAs when living in college latitudes whereas terrestrial and fully-aquatic mammals did non. To account for dietary influence, a double bond alphabetize was calculated including all FAs, and revealed no correlation with breadth in any of the groups.
Conclusions
Nosotros advise that FA modification is an of import component of the thermoregulatory strategy, particularly in semi-aquatic mammals. Potentially this is because, like terrestrial mammals, they experience the greatest air temperature variations across latitudes, only they lack a thick fur coat and rely primarily on their blubber. Unlike fully-aquatic mammals, extremely thick blubber is non platonic for semi-aquatic mammals, as this is detrimental to their manoeuvrability on land. Therefore, the adipose tissue in semi-aquatic mammals plays a more than of import role in keeping warm, and the modification of FAs becomes crucial to withstand cold temperatures and maintain a pliable blubber.
Groundwork
In spite of the thermal challenges of the aquatic medium, mammals take colonised this environs on seven carve up occasions and have sucessfully adapted [1]. Mammals exploit a wide range of thermal habitats, from the tropics to the poles, and from high altitudes to deep oceans; thus, they possess varied mechanisms to regulate their core body temperature. Changes in body size [2], pilus density [3], metabolic rate [four], blubber thickness [5, 6], and blubber thermal electrical conductivity [7] are some of these mechanisms, which have been well studied. However, the importance of the biochemical composition of the adipose tissue to mammalian thermoregulatory strategy has received less attending and however is likely to be of import, especially where mammals use blubber as a thermal barrier.
Unlike other tetrapods, mammals have different insulation strategies, from fully furred through to totally naked forms, each with different thermal benefits and constraints. We propose that where mammals do not use fur, the adipose tissue is expected to play a more important role in keeping warm. In blank-skinned mammals, the cooling of the torso surface in contact with air or h2o is of critical importance in social club to reduce the heat lost to the surroundings. In cold superficial tissues occurs peripheral vasoconstriction, where the flow of warm claret is reduced [8], thus reducing body heat loss and creating a thermal slope through the adipose tissue.
But cooling the body surface to temperatures like to those of the ambient could create a potential problem. In extremely cold environments, low temperatures could cause rigidity and solidification of superficial tissues; potentially it is here when the biochemical structure of fats becomes more of import.
Adipose tissues contain fat stored by and large in the form of triacylglicerol, which contains three molecules of fatty acids (FAs) [nine]. The degree of desaturation of a FA is given by its number of double bonds within the chain [10]. Every bit the degree of desaturation increases the tissues become more than fluid and can reach colder temperatures for longer periods without solidifying [11]. An adipose tissue layer with college degree of FA desaturation (FAs containing double bonds) is, therefore, better suited for lower temperatures every bit it is more effective for the maintenance of a thermal gradient.
The greatest contributor to adipose tissue FA composition is direct deposition of FAs from diet [9]. Still, the FA composition of consumers rarely matches exactly that of their nutrition [12]; which suggests that there are other factors influencing FA metabolism. Since mammals have the ability to synthesize some of their FAs de novo or modify them [10], they do not necessarily have a direct dietary origin. Thus, it is possible to distinguish between dietary and endogenous FAs [13]. Dietary FAs are those that could but be derived from the diet as animals are unable to synthesize them, such every bit about polyunsaturated FAs (PUFAs) [14, 15]. Endogenous FAs are those that tin exist readily synthesized by the animal; almost of them are saturated (SFAs) and monounsaturated FAs (MUFAs), although some of them may be partially obtained from diet [13, xiv].
Although the backdrop of the adipose tissue will largely depend on the type of diet consumed, there is some evidence that supports the idea of a thermal influence in the metabolism of FAs. For case, northern aquatic and terrestrial mammals have higher proportions of MUFAs in their cold extremities compared to the balance of the body [15], suggesting that FA desaturation takes place when tissues are maintained at low temperatures to avoid solidification. Similarly, 13-lined ground squirrels exhibit higher MUFA-to-SFA ratios in their adipose tissues in winter compared to summer [sixteen]. This suggests that certain changes in the FAs making up the adipose tissue are not only driven by nutrition merely also by thermal acclimatization [15, 17,18,xix]. The aim of this study was to evaluate whether thermal forces tin can have an result on endogenous FAs in mammals. Nosotros hypothesise that animals living in colder environments, or those animals non relying on a fur coat, will have college levels of FA desaturation in their adipose tissues.
To appraise the hypothesis of thermal influence in FAs, we investigated the differences in degree of FA desaturation across a total of 54 mammalian species from terrestrial, semi-aquatic and fully-aquatic environments. We also investigated the patterns of FA desaturation across mammals inhabiting different latitudinal regions. The direct upshot of ambient temperature is difficult to assess, since in that location is variation with season, water depth, and additionally, semi-aquatic mammals apply two environments where depending on their lifestyle they can spend substantially more fourth dimension in one than another. Therefore, in this report breadth was used as a coarse proxy for ambient temperature.
Fur-covered animals have skin temperatures closer to their trunk core whereas hairless animals have skin temperatures closer to those of the surrounding environment [twenty]. As most semi-aquatic mammals rely on a combination of both fur and blab [nineteen]; we investigated whether their FA desaturation is related to pilus density.
Nosotros report here the patterns of FA desaturation as a function of breadth, in mammals inhabiting different physical environments, and discuss the potential drivers.
Methods
Fatty acid data
In total, we included n = 54 mammalian species in this assay. A database of FA composition for mammalian species (n = 49 species) was collated from the literature, sourced from the online databases ScienceDirect and Google Scholar, using the search terms 'fatty acids', 'lipids', 'adipose tissue', 'fat' and 'blab'. Where available, but the FA values of the outer blubber layer were used, as FAs within this layer are less influenced past nutrition [12, 18, 21]. Where data was obtained from juveniles and adults, only adults data was used. When FA values of females and males were reported separately, only values from males were used, as females may feel changes in FAs associated with pregnancy and lactation (Boosted file 1: Table S1.). For terrestrial mammals, merely white adipose tissue data were used.
The FA composition from other 5 species (three otariids: subantarctic fur seal, Arctocephalus tropicalis; New Zealand fur seal, Arctocephalus forsteri; and Australian sea panthera leo, Neophoca cinerea; and 2 cetaceans: pygmy right whale, Caperea marginata; and Risso'southward dolphin, Grampus griseus) was analysed from 0.3 g samples of outer blab, using the SM Budge, SJ Iverson and HN Koopman [ix] method to extract total lipids and ready FA methyl esters. Gas chromatography analysis was performed as described in AI Guerrero and TL Rogers [22]. Blubber samples were sourced from animals stranded along the coast of Sydney, Australia. The FA composition of these species is available in Additional file 2: Tabular array S2.
Fatty acrid desaturation
To decide the degree of desaturation of endogenous FAs we calculated a desaturation index (∆ix-DI). This indicates to what extent potentially endogenous MUFAs could have been synthesized past modification of their corresponding SFAs [19]. PUFAs were non included in this assay, as about accept a dietary origin [fifteen]; therefore differences in proportions of PUFAs are likely to be a result of differences in diet. Using the percentage by weight per FA, a ∆nine-DI was calculated according to the formula of A Käkëla and H Hyvärinen [xv]:
$$ \mathbf{\Delta }\mathbf{9}-\mathbf{DI}\kern0.5em =\frac{\left(\mathbf{wt}\%\mathbf{14}:\mathbf{1}\boldsymbol{\upomega } \mathbf{5}+\mathbf{wt}\%\mathbf{xvi}:\mathbf{1}\boldsymbol{\upomega } \mathbf{7}+\mathbf{wt}\%\mathbf{sixteen}:\mathbf{1}\boldsymbol{\upomega } \mathbf{9}+\mathbf{wt}\%\mathbf{xviii}:\mathbf{one}\boldsymbol{\upomega } \mathbf{9}+\mathbf{wt}\%\mathbf{18}:\mathbf{one}\boldsymbol{\upomega } \mathbf{7}\right)}{\left(\mathbf{wt}\%\mathbf{14}:\mathbf{0}+\mathbf{wt}\%\mathbf{16}:\mathbf{0}+\mathbf{wt}\%\mathbf{eighteen}:\mathbf{0}\correct)} $$
where wt%, is the per centum past weight of the corresponding FA.
To account for the event of dietary FAs on the desaturation of fat tissues, nosotros calculated a double bond index (DBI), which includes all MUFAs and PUFAs, using the following formula [23, 24]:
$$ DBI=\frac{\sum x\left( wt\%\correct)}{100} $$
where wt%, is the per centum by weight of each FA and ten is its number of double bonds.
Explanatory variables
The traits environment and latitude were collected for all 54 species in the database. Surroundings was categorised equally terrestrial, semi-aquatic or fully-aquatic; where terrestrial mammals (northward = xv) are those whose complete life cycle takes place on country only; semi-aquatic species (due north = 25) are those who rely on both land (or water ice) and water for breeding and feeding; and fully-aquatic species (n = xiv) are those who spend their entire lives in water. Latitude corresponds to the location where the blubber samples were collected.
Hair density values, number of hairs per area (mmtwo), were obtained from the literature for near semi-aquatic mammals (n = 22), using the search terms 'fur density' and 'hair density' plus the species name, on ScienceDirect and Google Scholar. When primary and secondary hair densities were calculated separately, we added them upward and used total hair density. Values were log-transformed for analysis.
Data assay
Nosotros used a model choice approach to test whether factors could explain the variation of ∆nine-DIs (potentially endogenous FAs) or DBI (total FAs) in mammals. Initially, with the entire dataset (northward = 54 species), nosotros included the factors environs, latitude, or a combination of these variables, so, with a smaller dataset of 22 semi-aquatic mammals nosotros included the factors latitude, fur density, or a combination of these variables.
In detail, for the entire mammalian FA desaturation dataset (∆9-DI or DBI) we tested the post-obit models: (a) an additive model containing the two variables (β0 + βenvironment + βlatitude); (b) an surroundings and latitude interaction model (β0 + βenvironment*βlatitude); models where each variable would be the sole predictor for the desaturation of FAs: (c) a latitude model (β0 + βbreadth) or (d) environment model (β0 + βsurroundings); and (east) a Nada model (β0).
Some other series of models tested the relationship between pilus density, latitude and ∆9-DI in semi-aquatic mammals. For this data subset, we tested: (a) an additive model containing two variables (β0 + βlatitude + βhair_density); (b) models to test for different slopes of pilus density with latitude (β0 + βlatitude*βhair_density); and (c) a hair density model (β0 + βhair_density), (d) a latitude model (β0 + βlatitude) and (east) a Aught model (β0).
To identify the model(s) with best support, we calculated the Akaike'due south information criterion (with a correction for sample size; AICc) and the Akaike weight [25,26,27] for each model.
To account for the potential that phylogenetic relatedness confounds the variation in mammalian ∆9-DIs or DBI, we used phylogenetic generalised least squares (PGLS) analysis [28]. PGLS uses a lambda (λ) value, an estimate of phylogenetic correlation, that varies between 0 (indicating phylogenetic independence) and i (indicating that variation of traits between species covary proportionally with their phylogenetic relatedness) [29, xxx]. We used the S Faurby and J-C Svenning [31] mammalian supertree and included 1000 random iterations to resolve possible polytomies. Two trees were pruned to include only the species present in our dataset, the first including all mammals for which we had data (due north = 54), the second tree including only the semi-aquatic mammals for which hair density data was available (n = 22). The R (version three.0.1) [32] packages, 'phytools' [33] and 'caper' [34] were used for tree manipulation and PGLS analyses, respectively. For models with the highest back up, we extracted the 95% confidence intervals (CIs) and significance was deemed when the CIs did not overlap 0.
In order to identify which individual FAs drive changes in desaturation, when ∆9-DI was found to be significantly correlated with latitude, we applied linear regression analyses to those individual SFAs and MUFAs used to calculate the ∆9-DI.
Stratification of blubber FAs has been reported for most marine mammals [22] where the outer layer usually has greater proportions of MUFAs and smaller amounts of SFAs compared to the inner layer. Thus, to determine whether the use of different blubber sections (whole blubber core vs outer layer) would affect our analyses, we run an independent PGLS analysis for pinniped species for which either the outer layer (n = fourteen) or the whole blubber (due north = six) was used. We did non do the same for fully-aquatic mammals, as most FA values were obtained from the outer layer, with just ii exceptions (Additional file 2: Table S2.).
Values are presented as hateful ± standard deviation (SD) unless otherwise noted.
Results
Desaturation of endogenous fatty acids
The desaturation of endogenous FAs, ∆9-DI, was best explained by the interactive model (Tabular array 1) including surroundings and latitude (β0 + βenvironment*βlatitude). This model received the most support and deemed for 33% of the variance (r = 0.57), although the condiment model including environs and breadth (β0 + βenvironment + βlatitude) had ∆AICc values inside two units, which suggests that this model was equally supported. The Akaike weights showed that the interactive model was 2.7 times more than probable to exist the driver of mammalian ∆9-DI than the additive model. The PGLS λ value was 0.47; suggesting that the variation of ∆9-DI is not entirely dependent of phylogeny.
Fully-aquatic mammals have a mean ∆9-DI of three.08 ± 1.07, whereas semi-aquatic mammals take a ∆nine-DI of 3.09 ± one.75, and terrestrial mammals take a lower ∆9-DI of 1.37 ± 0.70. Among the three environments, just the ∆9-DI of semi-aquatic species had a significant positive correlation (CIs do non overlap 0) with latitude (slope = 0.06, CIs = 0.02, 0.11), such that semi-aquatic mammals at college latitudes have significantly greater Δ9-DI values than species at lower latitudes (Fig. 1b). In comparison, the Δ9-DI values of fully-aquatic (gradient = 0.02, CIs = − 0.02, 0.05) and terrestrial (slope = − 0.01, CIs = − 0.08, 0.05) mammals do not testify a relationship with latitude (Fig. 1a and c).
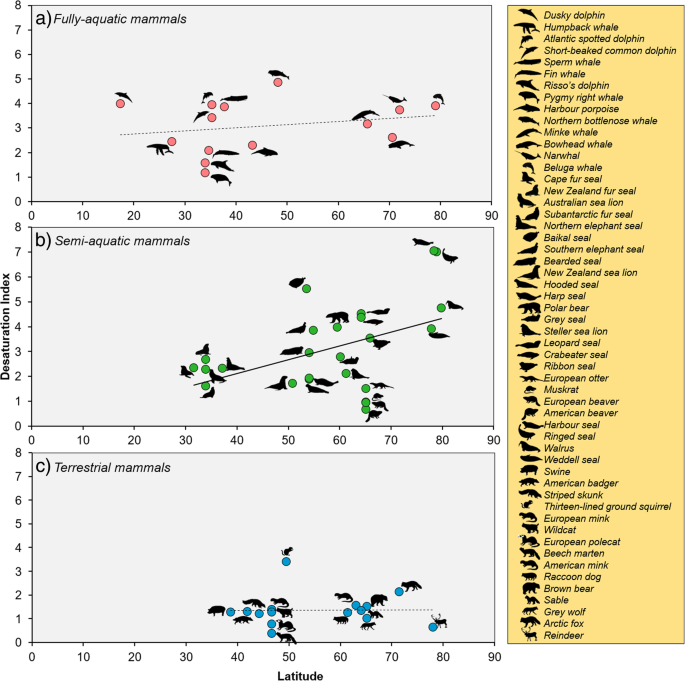
Desaturation index of fatty acids as a function of breadth for mammals inhabiting dissimilar environments. Dashed (not-significant correlation) and solid (significant correlation) lines indicate the phylogenetic generalised to the lowest degree squares (PGLS) regression lines for: a fully-aquatic (y = 0.02(x) + ii.27;n = 14); b semi-aquatic (y = 0.06(ten) − one.34;n = 25); and c terrestrial mammals (y = − 0.01(x) + i.62;n = fifteen). Just the blab of semi-aquatic mammals displayed significantly (CIs = 0.02, 0.11) higher fat acid desaturation when living in colder latitudes. Silhouettes are listed in the same lodge as they appear in each plot, from left to right and pinnacle to bottom. Scientific names can be found in Additional file 1: Table S1
Pinnipeds for which merely the outer layer was used, still displayed a significant correlation between Δ9-DI values and breadth (slope = 0.07, CIs = 0.04, 0.11; Fig. two); however, those pinniped species for which the whole blubber core was used, displayed a non-significant just positive correlation between Δ9-DI and latitude (slope = 0.03, CIs = − 0.03, 0.09).
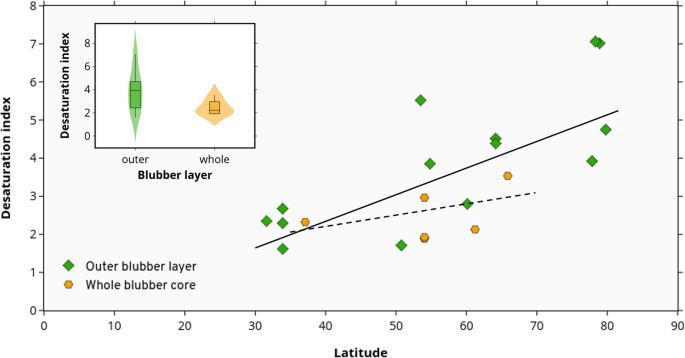
Desaturation index of blubber fatty acids as a function of breadth for pinnipeds, separated by blubber department used for analyses: whole blubber cadre (from pare to muscle; n = 6) or only the outer layer (section merely beneath the peel; n = 14). Based on phylogenetic generalised least squares (PGLS), the dashed regression line indicates a non-significant correlation for whole blab fatty acids (y = 0.03(x) + 1.01) whereas the solid line indicates significant correlation for outer blab fatty acids (y = 0.07(x) − 0.41)
Desaturation of total fatty acids
The DBI including all dietary and endogenous desaturated FAs was best explained past the environment model (β0 + βenvironment), which received the most support, explaining 50% of the variance (r = 0.seventy). The other model with ∆AICc value within 2 units was the additive model (β0 + βsurroundings + βbreadth), which suggests that this model is equally supported; still, the improver of latitude only explains an additional variance of 0.1%. In addition, environs model was 1.8 times more likely to be the driver of DBI in mammals than the additive model (Table ane). The PGLS λ value was closer to 1 (0.70), suggesting that DBI covaries in proportion to the caste of shared evolutionary history.
Fully-aquatic mammals have a mean DBI of 1.25 ± 0.36, whereas semi-aquatic mammals accept a DBI of i.61 ± 0.36 and terrestrial mammals a lower value of 0.85 ± 0.eighteen (Fig. iii). Intercept values were significantly different between fully-aquatic and terrestrial mammals (CIs = − 0.67, − 0.18), and between fully-aquatic and semi-aquatic mammals (CIs = 0.11, 0.55).
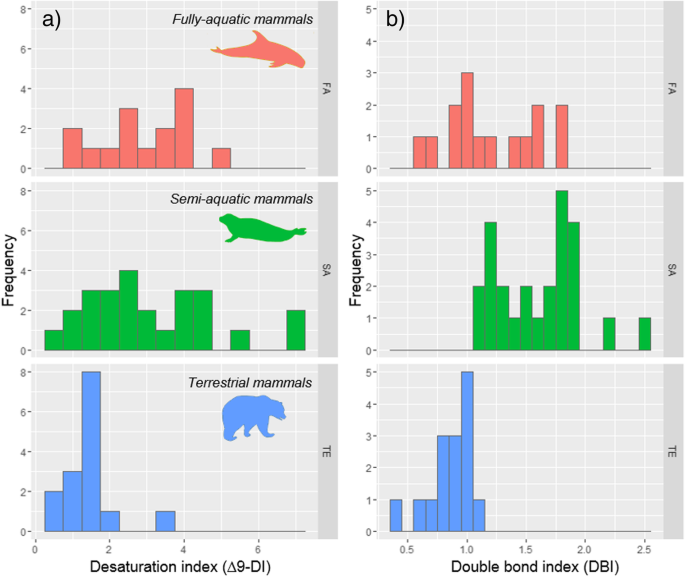
Histogram of a desaturation (∆9-DI) and b double bail index (DBI) of terrestrial (TE), semi-aquatic (SA), and fully-aquatic (FA) mammals. DBI has been calculated using all monounsaturated and polyunsaturated fatty acids, whereas ∆ix-DI was calculated using but endogenous fat acids
Effect of hair density and latitude on fatty acids of semi-aquatic mammals
When we included hair density every bit a variable for the ∆9-DI of semi-aquatic mammals, nosotros found that the model with the best support was the latitude model (β0 + βlatitude, Table 2), which explains 35% of the variance (r = 0.lx). The PGLS λ was 0.45; and there were no other models within two ∆AICc units. Including these 22 species, there was a positive meaning correlation between latitude and ∆9-DI (gradient = 0.08, CIs = 0.03, 0.12). Pilus density just explained an additional two% of the variance in the second-best model (β0 + βhair_density + βlatitude), which had an ∆AICc of two.5.
Variation of individual fat acids with latitude
Linear regression assay was only applied to pinniped species, every bit the correlation between ∆9-DI and latitude seems to be a pinniped-only pattern. Most MUFAs increased significantly (P < 0.01) with breadth whereas most SFAs (P < 0.01) decreased significantly (Fig. four). Only the FAs 14:0 (P = 0.98) and 18:1ω9 (P = 0.93) did not show variation with breadth.
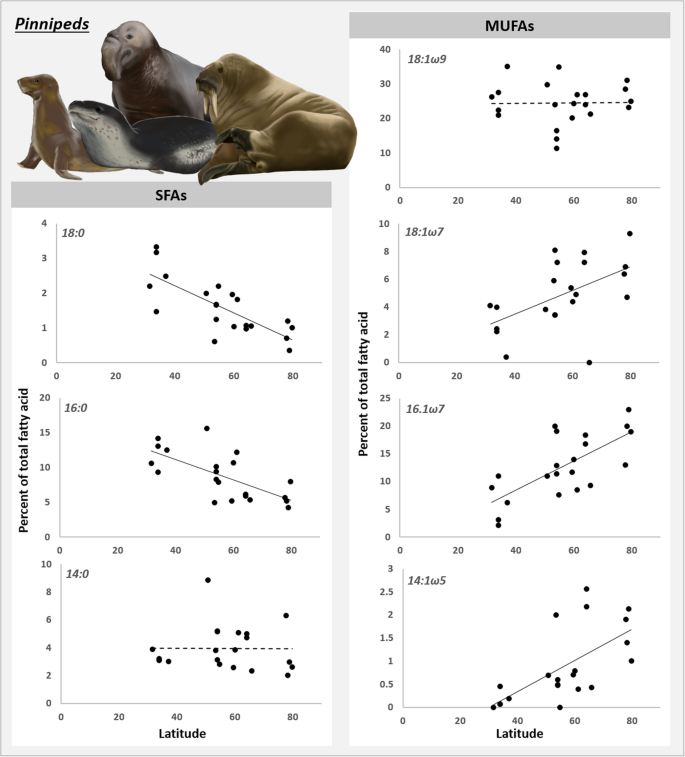
Percentage of saturated (SFAs) and monounsaturated (MUFAs) fatty acids as a office of latitude, for pinniped species. Dashed tendency lines indicate no meaning correlation betwixt latitude and percent of fat acid, and solid lines signal significant correlation. These fat acids were used to calculate the desaturation index
Discussion
Nosotros prove that the environment is potentially an of import commuter of FA desaturation in mammals. The variable environment was consistently important whether dietary FAs were considered (DBI), or non (∆9-DI). Conversely, latitude was an important commuter only for ∆ix-DI. The latitude in which the mammal lived was important for semi-aquatic mammals merely information technology was not for terrestrial and fully-aquatic mammals. This study provides insights to whether this is only because the surroundings offers different FAs through its food webs, or also due to the demands each environment imposes which leads to intrinsic modification of FAs.
Furnishings of breadth and environment on fatty acids
The best model of ∆9-DI included an interaction between surroundings and latitude. Aquatic mammals had higher degrees of desaturation than terrestrial mammals (Fig. 1), as expected. The aquatic medium has higher cooling potential than air [35], which suggests that the biochemical composition of adipose tissues plays a more important part in thermoregulation in aquatic mammals. Additionally, aquatic mammals use blubber every bit principal insulator whereas most terrestrial species use fur.
In fully-aquatic mammals, although there was a pocket-sized positive correlation between ∆9-DI and latitude, this was not pregnant (Fig. 1a). Because fully-aquatic mammals rely solely on their blubber every bit a thermal insulator [nineteen], this outcome was not expected. In bare-skinned animals, the skin and superficial tissues become very common cold when they are exposed to low environmental temperatures [4]. In whales, for case, the skin temperature has been institute to equal that of the water [twenty]. Therefore, the question is, why do fully-aquatic mammals not display greater degree of FA desaturation when they live in higher (colder) latitudes?
There are limitations to the written report that could help us sympathize the absence of a pattern. Although the relationship between desaturation and latitude was positive, it was not significant, which tin can exist due to the small sample size. In addition, we used whole blubber FAs for two of the species living in the highest latitudes: the narwhal, Monodon monoceros, and the beluga whale, Delphinapterus leucas (Fig. i); whereas for all other fully-aquatic species we used the outer blubber layer. Since the blubber of marine mammals is stratified, where MUFAs are more arable in the outer layer and SFAs in the inner layer [22], the use of whole blubber FAs should reduce the MUFA-to-SFA ratio leading to a decrease in ∆ix-DI. If nosotros had the data of the outer layer ∆9-DI instead, the values should be college which could take led to a pregnant correlation. Unfortunately, it is difficult to measure out the magnitude of the outcome of the blubber section utilised, due to the small sample size.
Still, there is an aspect of FA composition not accounted for in this study. Certain families of cetaceans synthesise considerable levels of the endogenous isovaleric acrid, a brusque branched-concatenation FA [36]. This FA has an extremely depression melting point of − 37.6 ºC [37], and it has been found in great quantities (e.k.: ~ 35% in Hector's dolphin, Cephalorhynchus hectori) in the outer blab layer of some odontocetes [36]. They are hypothesised to play a part in maintaining blubber pliability in common cold environments as they have been establish to be more abundant in species inhabiting colder waters [9, 36]. Therefore, potentially some fully-aquatic mammals would rather synthesise this or other branched-chain FAs than increment their desaturation levels. Withal, the synthesis of this FA is non ubiquitous among cetaceans; therefore, this does not completely explain the niggling variation of desaturation with breadth.
However; do fully-aquatic mammals demand to maintain a pliable blab in cold environments? The need for a pliable blubber might non exist such for cetaceans. Some toothed whales (e.m. beaked and sperm whales) take FAs in the course of wax esters instead of triacylglycerols [38]; these wax esters have relatively high melting points [39], which makes them solid at most of the temperatures they discover in the surrounding water. This implies that these species have rather rigid blubber layers, which does not seem to be an inconvenient for them.
Terrestrial mammals displayed low values of ∆9-DI and DBI, compared to the other groups (Fig. 3) and they did not exhibit a relationship between ∆9-DI and latitude (Fig. 1c). Although terrestrial mammals living in arctic climates see swell diurnal and seasonal temperature variations [40], they seem to have piffling demand for lowering the solidifying point of their adipose tissue. Our observations agree with those of PF Scholander, V Walters, R Hock and L Irving [41], who state that trunk fatty does not seem to play any function in the insulation of terrestrial arctic mammals. In fact, fur has been found to be a more than efficient insulator than adipose tissue [41], since information technology creates the same thermal gradient between body core and body surface, merely across a much smaller thickness [19].
In the case of animals with poor insulating fur (thin, sparse fur or hairless), the thermal role of adipose tissues is expected to gain importance. V Henriques and C Hansen [42] compared groups of pigs living at 0 °C: one group with their skin direct exposed to the cold ambience air and the other where animals had a sheepskin garment. Later on 2 months, bare-skinned pigs had adipose tissues that solidified at a temperature ii.4 °C lower than pigs protected past a coat. This suggests an increment in the degree of desaturation in the FAs of pigs whose skin was in direct contact with the cold environment. Both groups were fed the same nutrient; therefore, this could be either a issue of the modification of their FAs or the selective incorporation of sure FAs from the diet to fulfil their thermal needs. Depression tissue temperatures, therefore, seem to touch the desaturation of FAs. Conversely, when the peel temperature is maintained close to the warm body temperature, as it is the case of garment-covered pigs and fur-covered mammals, a modification of FAs may not be necessary.
Semi-aquatic mammals, unlike the other two groups, have higher ∆9-DI equally they inhabit college latitudes (Fig. 1b). When hair density was included in the analysis, latitude was yet a more of import driver of ∆9-DI (Table 2). Conversely, DBI, which included dietary FAs, did not change with breadth, which suggests that this human relationship betwixt desaturation of FAs (∆9-DI) and latitude is not due to differences in diet.
The blubber section used for assay seems to affect at some degree the ∆nine-DI, equally values of whole blubber cores where usually lower than those of outer layer, for animals living in similar latitudes. Thus, when simply the outer layer was used, the correlation between ∆9-DI and latitude for pinnipeds is stronger, with a steeper gradient (Fig. 2). However, since the number of data points obtained from whole blubber cores was relatively small (n = vi, versus n = 25 for all semiaquatic species), this did not change the correlation institute betwixt ∆nine-DI and breadth in semiaquatic mammals.
Figure 4 allows u.s.a. to infer some of the mechanisms behind the increased FA desaturation in colder regions. The FAs xviii:1ω9 and fourteen:0 did non display a relationship with latitude, suggesting that they practice not have an important role in the increased FA desaturation of pinnipeds exposed to colder temperatures. The other FAs, however, behaved as expected; the SFAs xvi:0 and 18:0 decreased with latitude whereas the MUFAs xiv:1ω5, sixteen:1ω7 and eighteen:1ω7 increased.
The outcome of temperature on FAs has been well documented for organisms at lower trophic levels. The almost ordinarily observed change in FAs post-obit a temperature shift is an amending in desaturation. In bother fish, for example, the expression of the enzyme that incorporates the first unsaturation bond into saturated FAs, Δ9-desaturase, is induced past cold temperature [43]. Similarly, an increase of FA desaturation when exposed to lower ecology temperatures has been reported for algae and copepods [44,45,46]. Since mammals exert sensitive command over their desaturases [47], cold-induced mechanisms could be regulating their increase of MUFAs and decrease of SFAs at higher latitudes. Although this wide analysis does not allow u.s.a. to determine the biochemical paths backside these patterns; we expect that these findings encourage further investigation on the thermoregulatory mechanisms of blab.
Therefore, why are semi-aquatic mammals the just group that modifies its FAs intrinsically every bit a response to ambient temperatures? Semi-aquatic mammals are the only ones that move between two environments. Switching from one environs to another implies that animals are exposed to contrasting temperatures and very different oestrus loss rates. Additionally, they possess both fur and blubber insulation, which is an interesting aspect of mammalian thermoregulation. The presence of dense, waterproof fur is a characteristic of recent entries to the aquatic environment, such as otters, beavers and other rodents [19, 48]. In this written report, muskrats, beavers and otters, although living in common cold regions, were the species with the lowest degrees of desaturation (Fig. 1b) suggesting that this is a pinniped-only phenomenon. Interestingly, these recent entries to the aquatic medium have the highest hair densities (See Boosted file 1: Tabular array S1.). This supports the idea that these mammals rely on dense waterproof fur, rather than the adipose tissue, as an insulator [48]. The efficiency of this coat relies on the air trapped in its hairs [49], which forms a protective warm layer and keeps the pare relatively dry [50]. This thermal barrier, withal, can be a disadvantage in swimming performance.
Earlier entries to the aquatic environment, such every bit pinnipeds, take become much ameliorate swimmers, and this has unsaid the reduction of elevate through more streamlined bodies [51], reduction of fur density and thickness [52], and the transition to blubber as an insulator [19]. They possess a wettable fur that is not a proficient insulator in water, every bit the air layer trapped in the fur is released due to compression when diving [nineteen]; thus, blab becomes more important as a thermal barrier.
A thick blubber layer could ensure an constructive insulation in h2o, which is the case of most fully-aquatic mammals, but for a semi-aquatic mammal an extremely thick blubber layer tin can be problematic. Most semi-aquatic mammals are relatively modest compared to cetaceans, and an extremely big blubber layer would impede an agile terrestrial locomotion, especially for those animals living in rookeries with steep slopes, which is common amongst sea lions and fur seals. A trade-off, having a not as well thick, but efficient thermal insulator is therefore imperative. A variation in FA composition can be an effective mechanism to maintain a good insulating layer without limiting the manoeuvrability of these animals. Higher FA desaturation in higher latitudes allows the blubber layer most the peel to lower its temperature and therefore reduce rut loss to the surrounding environment due to peripheral vasoconstriction. This characteristic can be more important in pinnipeds than in whatever of the other two groups, every bit they cannot afford a very thick pelage similar terrestrial mammals, and neither tin they develop extremely thick blubber equally fully-aquatic mammals.
Additionally, air temperature variations could explicate why semi-aquatic mammals are significantly influenced by latitude and fully-aquatic mammals are not. The water temperature may vary widely, from 30 °C to about − 2 °C, from the tropics to the poles, whereas air temperature in the poles can hands drop to about − 25 °C in winter [53]. Therefore, semi-aquatic mammals are experiencing the most extreme temperature variations and, in absenteeism of an insulating fur coat, the modification of FA desaturation in college latitudes is a practiced solution to avoid tissue solidification.
Another interesting aspect of blubber is that it has been reported to display thermal behaviour consequent with phase change materials [54]; thus, using biochemical bonds to store and release heat when lipids melt or solidify at certain temperatures [55, 56]. Many of the FAs found in the blubber are classified every bit phase alter materials [57]; they have melting points betwixt 29° and 38 °C [55] which are inside mammalian body temperatures. This suggests that changes in desaturation of FAs might non only be related to pliability but also thermal storage; however, this needs further investigation.
Dietary influence
The influence of nutrition in the FA composition of mammals is undeniable, and this is why the use of FAs every bit trophic markers is gaining scientific attraction [14, 58]. However, there are many factors that tin affect the composition of FAs and we testify here that the event of thermal acclimatization is potentially one of them.
To calculate ∆9-DI, we used simply those FAs that tin be intrinsically synthesised; however, some FAs can be partially endogenous and partially dietary [14]. This is a limitation to this type of analysis. It can be argued that diet still has an influence in the desaturation of FAs. A recent study by SK Abbott, PL Else, TA Atkins and AJ Hulbert [59] shows that rats fed 12 diets showed changes in their MUFA content. In this study, when rats were fed fat that was high in MUFAs the adipose FA composition became increasingly higher in MUFAs, and vice versa. Even so, at that place are other examples where diet has had niggling influence on MUFAs and SFAs. For instance, in humans, PUFAs show a close human relationship between dietary intake and adipose tissue whereas SFAs and MUFAs are less closely correlated [60]. Radio-labelling studies showed that grey seals transformed SFAs into MUFAs because the radio-labelled palmitic acid 16:0 they were fed was modified into 16:1 in their blubber [61]. Other authors agree that MUFAs and SFAs are not expected to reverberate dietary intake [62]. In this study, there could still exist a dietary influence in the FA desaturation of mammals, even so this multi-species approach allows us to see that, when examined more broadly, at that place is an interesting influence of thermoregulation on FAs and that this is a fertile area to investigate further.
Conclusions
Nosotros show that the ability to change FAs is an important office of mammalian thermal plasticity, specially for mammals living under adverse thermal weather. We demonstrate that fully-aquatic mammals have increased FA desaturation in their blubber compared to terrestrial mammals, but they do not modify their FAs in different latitudes; they are potentially regulating other parameters of blubber instead. Terrestrial mammals rely mainly on fur as insulator; thus, their adipose tissues have low levels of FA desaturation which does non vary in colder latitudes. Semi-aquatic mammals increase their FA desaturation when living in colder regions, so that they can cool their superficial tissues near freezing temperatures without solidification. The ability to alter their FAs is imperative in semi-aquatic species, as they cannot afford to have extremely thick blab layers as fully-aquatic mammals since this would compromise their ability to move on state. Additionally, they are exposed to the greatest temperature variations, just similar terrestrial mammals, only they lack an insulating fur glaze and, therefore, modify their blubber biochemical composition.
Availability of data and materials
All information supporting the conclusions of this commodity are within the paper and its supporting information files.
Abbreviations
- ∆9-DI:
-
Desaturation index
- AICc:
-
Akaike information benchmark (corrected for sample size)
- CIs:
-
Conviction intervals
- DBI:
-
Double bond index
- FA(due south):
-
Fatty acid(southward)
- MUFA(southward):
-
Monounsaturated fat acid(s)
- PUFA(due south):
-
Polyunsaturated fatty acrid(southward)
- SFA(due south):
-
Saturated fatty acid(s)
References
-
Uhen MD. Evolution of marine mammals: Back to the sea after 300 one thousand thousand years. Anat Rec. 2007;290(6):514–22.
-
Meiri South, Dayan T. On the validity of Bergmann's rule. J Biogeogr. 2003;30(iii):331–51.
-
Cooper CE. Biophysical properties of the pelt of a diurnal marsupial, the numbat (Myrmecobius fasciatus), and its role in thermoregulation. J Exp Biol. 2003;206(sixteen):2771–7.
-
Irving L, Hart JS. The metabolism and insulation of seals equally bare-skinned mammals in cold water. Can J Zool. 1957;35(4):497–511.
-
Castellini MA, Trumble SJ, Mau TL, Yochem PK, Stewart BS, Koski MA. Body and blubber relationships in antarctic pack ice seals: implications for blubber depth patterns. Physiol Biochem Zool. 2009;82(2):113–20.
-
Pabst DA, Rommel SA, WA ML. The functional morphology of marine mammals. In: Reynolds JE, Rommel SA, editors. Biology of marine mammals. Melbourne: Melbourne University Press; 1999. p. 15–72.
-
Kvadsheim PH, Folkow LP, Blix Every bit. Thermal conductivity of minke whale blubber. J Therm Biol. 1996;21(ii):123–8.
-
Elsner R. Living in water: solutions to physiological issues. In: Reynolds JE, Rommel SA, editors. Biology of Marine Mammals. Washington, D.C.: Smithsonian Establishment Printing; 1999. p. 73–116.
-
Budge SM, Iverson SJ, Koopman HN. Studying trophic environmental in marine ecosystems using fatty acids: a primer on analysis and estimation. Mar Mamm Sci. 2006;22(4):759–801.
-
Miyazaki Grand, Ntambi JM. CHAPTER 7 - Fatty acid desaturation and chain elongation in mammals A2 - Vance, Dennis Due east. In: Vance JE, editor. Biochemistry of Lipids, Lipoproteins and Membranes. 5th ed. San Diego: Elsevier; 2008. p. 191–211.
-
Irving Fifty, Schmidt-Nielsen K, Abrahamsen NS. On the melting points of animal fats in cold climates. Physiol Zool. 1957;30(2):93–105.
-
Grahl-Nielsen O, Haug T, Lindstrøm U, Nilssen KT. Fatty acids in harp seal blubber do non necessarily reflect their diet. Mar Ecol Prog Ser. 2011;426:263–76.
-
Iverson SJ, Field C, Bowen WD, Blanchard Due west. Quantitative fatty acrid signature assay: a new method of estimating predator diets. Ecol Monogr. 2004;74(ii):211–35.
-
Iverson SJ. Milk secretion in marine mammals in relation to foraging: tin milk fatty acids predict diet? In: Boyd IL, editor. Marine mammals: advances in behavioural and population biology. Oxford: The Zoological Society of London; 1993.
-
Käkëla A, Hyvärinen H. Site-specific fatty acid composition in adipose tissues of several northern aquatic and terrestrial mammals. Comp Biochem Physiol A. 1996;115(iv):501–14.
-
Price ER, Armstrong C, Guglielmo CG, Staples JF. Selective mobilization of saturated fat acids in isolated adipocytes of hibernating xiii-lined ground squirrels Ictidomys tridecemlineatus. Physiol Biochem Zool. 2013;86(2):205–12.
-
Harlow HJ, Varnell T. Wintertime changes in fatty acid composition of badger and coyote tissues. Comp Biochem Physiol B. 1980;67A:211–4.
-
Koopman HN. Phylogenetic, ecological, and ontogenetic factors influencing the biochemical structure of the blab of odontocetes. Mar Biol. 2007;151(1):277–91.
-
Liwanag HE, Berta A, Costa DP, Budge SM, Williams TM. Morphological and thermal backdrop of mammalian insulation: the evolutionary transition to blubber in pinnipeds. Biol J Linn Soc. 2012;107:774–87.
-
Hokkanen JE. Temperature regulation in marine mammals. J Theor Biol. 1990;145:465–85.
-
Guerrero AI, Negrete J, Márquez MEI, Mennucci J, Zaman K, Rogers TL. Vertical fatty acrid composition in the blubber of leopard seals and the implications for dietary assay. J Exp Mar Biol Ecol. 2016;478:54–61.
-
Guerrero AI, Rogers TL. Blab fatty acrid composition and stratification in the crabeater seal, Lobodon carcinophaga. J Exp Mar Biol Ecol. 2017;491:51–vii.
-
Richardson T, Tappel AL, Gruger EH. Essential fatty acids in mitochondria. Curvation Biochem Biophys. 1961;94(1):1–6.
-
Westward GC, Burns JJ, Modafferi Grand. Fatty acid composition of blubber from the 4 species of Bering Sea phocid seals. Can J Zool. 1979;57:189–95.
-
Garamszegi LZ, Mundry R. Multimodel-Inference in Comparative Analyses. In: Mod phylogenetic comparative methods and their application in evolutionary biological science: concepts and practise. Berlin, Heidelberg: Springer; 2014. p. 305–31.
-
Burnham KP, Anderson DR. Model option and multimodal inference: a applied information-theoretic arroyo. New York: Springer; 2002.
-
Martin Thousand, Tucker MA, Rogers TL. Does size thing? Examining the drivers of mammalian vocalizations. Development. 2017;71(2):249–60.
-
Laiolo P, Rolando A. The development of vocalisations in the genus Corvus: furnishings of phylogeny, morphology and habitat. Evol Ecol. 2003;17(two):111–23.
-
Freckleton RP, Harvey PH, Pagel M. Phylogenetic analysis and comparative information: a test and review of testify. Am Nat. 2002;160(6):712–26.
-
Pagel M. The maximum likelihood approach to reconstructing bequeathed graphic symbol states of discrete characters on phylogenies. Syst Biol. 1999;48(3):612–22.
-
Faurby Due south, Svenning J-C. A species-level phylogeny of all extant and belatedly 4th extinct mammals using a novel heuristic-hierarchical Bayesian approach. Mol Phylogenet Evol. 2015;84:fourteen–26.
-
Team RC: R: A linguistic communication and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014; 2016.
-
Revell LJ. Phytools: an R bundle for phylogenetic comparative biology (and other things). Methods Ecol Evol. 2012;3(2):217–23.
-
Orme D: The caper bundle: comparative analysis of phylogenetics and evolution in R. R package version 2013, 5(ii).
-
Nienaber J, Thomton J, Horning Grand, Polasek L, Mellish J-A. Surface temperature patterns in seals and sea lions: a validation of temporal and spatial consistency. J Therm Biol. 2010;35(8):435–forty.
-
Koopman HN, Iverson SJ, Read AJ. High concentrations of isovaleric acid in the fats of odontocetes: variation and patterns of accumulation in blubber vs. stability in the melon. J Comp Physiol B. 2003;173(three):247–61.
-
Fasman GD. Handbook of biochemistry and molecular biology. Lipids, carbohydrates, steroids-3; 1975.
-
Lockyer C. Body composition of the sperm whale, Physeter catodon, with special reference to the possible functions of fat depots: marine research establish; 1991.
-
Patel S, Nelson DR, Gibbs AG. Chemical and physical analyses of wax ester properties. J Insect Sci. 2001;1(1):1-vii.
-
Irving L, Peyton LJ, Bahn CH, Peterson RS. Regulation of temperature in Fur seals. Physiol Zool. 1962;35(4):275–84.
-
Scholander PF, Walters V, Hock R, Irving L. Body insulation of some Arctic and tropical mammals and birds. Biol Balderdash. 1950;99(2):225–36.
-
Henriques 5, Hansen C. Vergleichende Untersuchungen über dice chemische Zusammensetzung des thierischen Fettes1. Skandinavisches Archiv Für Physiologie. 1901;11(ane):151–65.
-
Tiku P, Gracey A, Macartney A, Beynon R, Cossins A. Cold-induced expression of Δ9-desaturase in carp past transcriptional and posttranslational mechanisms. Science. 1996;271(5250):815–viii.
-
Guschina IA, Harwood JL. Algal lipids and upshot of the environment on their biochemistry. In: Lipids in aquatic ecosystems. New York: Springer; 2009. p. i–24.
-
Thompson GA Jr. Lipids and membrane role in green algae. Biochim Biophys Acta. 1996;1302(1):17–45.
-
Jeffries HP. Seasonal composition of temperate plankton communities: fatty ACIDS1. Limnol Oceanogr. 1970;15(3):419–26.
-
Aguilar PS, De Mendoza D. Control of fatty acrid desaturation: a mechanism conserved from bacteria to humans. Mol Microbiol. 2006;62(half dozen):1507–fourteen.
-
Reynolds PS. Size, shape, and surface surface area of beaver, Castor canadensis, a semiaquatic mammal. Can J Zool. 1993;71(5):876–82.
-
Fish Iron, Smelstoys J, Baudinette RV, Reynolds PS. Fur does not fly, information technology floats: buoyancy of pelage in semi-aquatic mammals. Aquat Mamm. 2002;28(ii):103–12.
-
Dawson TJ, Fanning FD. Thermal and energetic issues of semiaquatic mammals: a study of the Australian water rat, including comparisons with the platypus. Physiol Zool. 1981;54(iii):285–96.
-
Fish F. Influence of hydrodynamic-design and propulsive mode on mammalian pond energetics. Aust J Zool. 1994;42(1):79–101.
-
Liwanag HE, Berta A, Costa DP, Abney M, Williams TM. Morphological and thermal properties of mammalian insulation: the development of fur for aquatic living. Biol J Linn Soc. 2012;106(iv):926–39.
-
Bargagli R. Antarctic ecosystems: environmental contamination, climatic change, and human affect, vol. 175. Berlin, Heidelberg: Springer Science & Business organization Media; 2006.
-
Singleton EM, McLellan WA, Koopman HN, Pokorny A, Scharf FS, Ann Pabst D. Lipid composition and thermal properties of the blubber of Gervais' beaked whale (Mesoplodon europaeus) across ontogeny. Mar Mamm Sci. 2017;33(2):695–705.
-
Suppes Thousand, Goff M, Lopes Due south. Latent rut characteristics of fat acid derivatives pursuant phase change fabric applications. Chem Eng Sci. 2003;58(9):1751–63.
-
Bagge LE, Koopman HN, Rommel SA, McLellan WA, Pabst DA. Lipid grade and depth-specific thermal properties in the blubber of the short-finned pilot whale and the pygmy sperm whale. J Exp Biol. 2012;215(24):4330–9.
-
Dunkin RC, McLellan WA, Blum JE, Pabst DA. The ontogenetic changes in the thermal backdrop of blubber from Atlantic bottlenose dolphin Tursiops truncatus. J Exp Biol. 2005;208(8):1469–fourscore.
-
Dalsgaard J, St. John M, Kattner G, Müller-Navarra D, Hagen W. Fatty acid trophic markers in the pelagic marine environment. Adv Mar Biol. 2003;46:225–340.
-
Abbott SK, Else PL, Atkins TA, Hulbert AJ. Fatty acrid composition of membrane bilayers: importance of diet polyunsaturated fat balance. Biochim Biophys Acta Biomembr. 2012;1818(5):1309–17.
-
Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation betwixt dietary intake and adipose tissue limerick of selected fatty acids in US women. Am J Clin Nutr. 1998;67(1):25–30.
-
Budge SM, Cooper MH, Iverson SJ. Demonstration of the deposition and modification of dietary fatty acids in pinniped blab using radiolabelled precursors. Physiol Biochem Zool. 2004;77(4):682–7.
-
Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fat acrid intake. Am J Clin Nutr. 2002;76(iv):750–7.
Acknowledgements
Nosotros would like to thank every member of the Mammal Lab at UNSW for their back up and helpful feedback on our manuscript, and especially Marlee Tucker and Kobe Martin for statistical communication. We admit the Bioanalytical Mass Spectrometry Facility within the Mark Wainwright Analytical Heart of the University of New South Wales, and peculiarly we give thanks Lewis Adler for his help with fatty acid analyses. This work has been conducted as function of Proyecto FONDECYT Postdoctorado N° 3180433.
Funding
This enquiry was conducted under the Australian Research Council Linkage Program LP0989933 to TR and FONDECYT Postdoctorado North° 3180433 to AG.
Author information
Affiliations
Contributions
TR and AG conceived the study. AG conducted laboratory analyses where required. TR and AG wrote the newspaper. Both authors read and approved the final manuscript.
Respective author
Ethics declarations
Ideals approval and consent to participate
Samples were nerveless opportunistically from dead stranded animals and provided to Tracey Rogers under NSW National Parks and Wildlife Service Scientific Research Licences 10455 and SL100217.
Consent for publication
Non applicable.
Competing interests
The authors declare that they accept no competing interests.
Boosted information
Publisher'southward Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Information collected beyond 54 mammalian species. Blubber section indicates whether samples analysed correspond to the whole cadre of blab or just the department closer to the skin (outer layer). Information sources are indicated for fat acid and hair density data. The desaturation index (∆9-DI) and Double bond index (DBI) calculated are also provided. (DOCX 87 kb)
Additional file 2:
Table S2. Fatty acid composition of five mammal species. Samples of the outer blubber layer were nerveless from stranded animals along the declension of Sydney, Commonwealth of australia. (DOCX 23 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution four.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted utilize, distribution, and reproduction in any medium, provided you requite appropriate credit to the original author(s) and the source, provide a link to the Artistic Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/i.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this article
Guerrero, A.I., Rogers, T.Fifty. From depression to high latitudes: changes in fatty acid desaturation in mammalian fat tissue advise a thermoregulatory role. BMC Evol Biol 19, 155 (2019). https://doi.org/ten.1186/s12862-019-1473-five
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s12862-019-1473-5
Keywords
- Adipose tissue
- Blab
- Environs
- Fur
- Insulation
- Breadth
- Acclimatization
- Macroecology
Source: https://bmcecolevol.biomedcentral.com/articles/10.1186/s12862-019-1473-5
Posted by: standifermustor.blogspot.com

0 Response to "Which Statement Best Relates Why These Animals Have So Much Blubber?"
Post a Comment